GMP-Compliant Contract Manufacturing for Global Pharmaceutical Markets
Sterile injectable products play a critical role in modern healthcare, particularly in acute care, critical therapies, and hospital-based treatments. As regulatory expectations increase and product complexity grows, pharmaceutical companies require manufacturing partners with advanced infrastructure, robust quality systems, and proven compliance capabilities.
Kilitch Healthcare (KHIL) offers sterile injectable manufacturing services designed to meet the stringent demands of the global pharmaceutical industry. Our GMP-compliant facilities support scalable, reliable, and high-quality pharmaceutical manufacturing for companies seeking dependable contract manufacturing solutions in India and international markets.
The Importance of Sterile Injectable Manufacturing in the Pharmaceutical Industry
Sterile injectables represent one of the most regulated segments of pharmaceutical manufacturing. These products require controlled environments, validated aseptic processes, and strict adherence to GMP in the pharmaceutical industry to ensure patient safety and therapeutic efficacy.
For pharmaceutical companies, outsourcing sterile manufacturing to a qualified partner enables access to specialized infrastructure while minimizing operational risk. A reliable sterile manufacturing partner ensures consistency, regulatory readiness, and supply continuity key requirements for hospital procurement teams and global healthcare partners.
Kilitch Healthcare’s Sterile Manufacturing Capabilities
Advanced Sterile Manufacturing Infrastructure
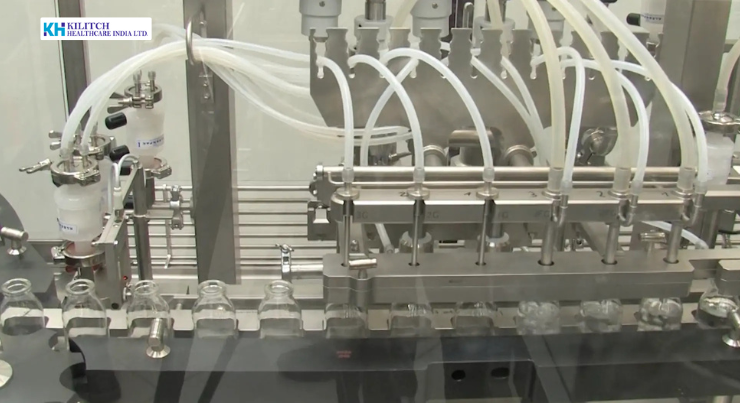
Kilitch Healthcare operates dedicated sterile manufacturing facilities designed to support injectable dosage forms under controlled aseptic conditions. Our infrastructure is built to align with international regulatory expectations and good manufacturing processes, ensuring high standards of quality and operational integrity.
Key facility features include:
- Classified cleanroom environments with controlled airflow systems
- Automated filling and sealing lines for sterile injectables
- Validated sterilization processes and aseptic handling protocols
- Environmental monitoring and contamination control systems
These capabilities enable KHIL to support pharmaceutical companies across multiple therapeutic segments while maintaining consistency and compliance.
End-to-End Sterile Injectable Manufacturing Workflow
Process Design and Technology Transfer
Each project begins with a structured technology transfer process, ensuring seamless integration of formulations, specifications, and manufacturing requirements. Our technical teams work closely with pharmaceutical companies’ research and development functions to ensure process reproducibility and scalability.
This collaborative approach supports pharmaceutical companies’ research and development objectives while maintaining regulatory alignment throughout the product lifecycle.
Aseptic Filling and Sterile Processing
Sterile injectables are manufactured using validated aseptic processes designed to minimize contamination risks. Our sterile manufacturing workflow incorporates:
- Controlled material and personnel movement
- In-process controls to maintain batch consistency
- Automated inspection and monitoring systems
These measures support reliable pharmaceutical manufacturing outcomes, particularly for hospital-grade and export-oriented products.
Quality Control and Batch Release
Comprehensive quality control testing is conducted at every stage of production. This includes physical, chemical, and microbiological testing in accordance with approved specifications and regulatory guidelines.
Only products that meet predefined quality standards are released, ensuring confidence for pharmaceutical companies, distributors, and healthcare institutions.
Compliance with Global GMP Standards
Regulatory Alignment and Quality Systems
Kilitch Healthcare’s manufacturing operations are governed by robust quality management systems aligned with GMP in the pharmaceutical industry. Our facilities follow structured documentation, validation, and audit processes to support compliance across regulated and semi-regulated markets.
Key compliance elements include:
- GMP-compliant documentation and batch records
- Equipment qualification and process validation
- Deviation management and corrective action systems
- Ongoing internal audits and quality reviews
This compliance-driven approach positions KHIL among reliable pharmaceutical manufacturing companies in India.
Business Advantages of Partnering with Kilitch Healthcare
Scalable Manufacturing Capacity
Our sterile manufacturing facilities are designed to support both small-batch and large-scale commercial production. This scalability allows pharmaceutical companies to respond to evolving market demands without additional capital investment.
Operational Efficiency and Risk Mitigation
By outsourcing sterile injectables manufacturing, pharmaceutical companies reduce the complexity associated with operating high-risk sterile facilities. KHIL manages manufacturing execution, compliance oversight, and quality assurance allowing partners to focus on commercialization and market expansion.
Reliable Supply for Institutional and Export Markets
Consistency and supply reliability are essential for hospital procurement teams and global distributors. Our structured production planning and validated processes ensure predictable supply chains and batch-to-batch consistency.
Supporting India’s Role in Global Pharmaceutical Manufacturing
India is recognized as a leading hub for pharmaceutical manufacturing, supported by a strong regulatory framework, skilled technical talent, and cost-efficient operations. As one of the best pharmaceutical companies in India offering contract manufacturing services, Kilitch Healthcare contributes to India’s reputation as a trusted global pharmaceutical partner.
Our sterile manufacturing services support pharmaceutical companies operating as:
- Domestic drug companies in India
- Export-focused pharmaceutical brands
- Multinational pharmaceutical organizations seeking Indian manufacturing partners
Why Kilitch Healthcare Is a Trusted Sterile Manufacturing Partner
Kilitch Healthcare combines technical expertise, regulatory discipline, and manufacturing reliability to support long-term partnerships. Our approach emphasizes:
- Quality-driven pharmaceutical manufacturing
- Transparent collaboration with clients
- Compliance-focused operational execution
- Long-term supply chain stability
These principles allow KHIL to function not just as a manufacturer, but as a strategic partner to pharmaceutical companies across global markets.
Applications Across Therapeutic and Institutional Segments
Our sterile injectable manufacturing services support a wide range of applications, including:
- Hospital and institutional supply
- Acute and critical care therapies
- Export-oriented injectable portfolios
- Contract manufacturing for branded and generic injectables
This versatility makes KHIL a preferred partner for pharmaceutical companies seeking dependable sterile manufacturing solutions.
Conclusion: Reliable Sterile Injectable Manufacturing for Global Healthcare
Sterile injectable manufacturing demands precision, compliance, and technical excellence. Kilitch Healthcare’s GMP-compliant facilities, structured workflows, and quality-focused culture provide pharmaceutical companies with a dependable manufacturing foundation.
By partnering with KHIL, pharmaceutical manufacturers gain access to scalable sterile manufacturing capabilities aligned with global regulatory expectations supporting growth, reliability, and long-term success in the pharmaceutical industry.
Contact Kilitch Healthcare
For pharmaceutical companies, healthcare brands, and global partners seeking a reliable sterile injectable contract manufacturing partner, Kilitch Healthcare offers the expertise, infrastructure, and compliance assurance required for today’s healthcare markets.
Connect with our team to discuss sterile injectable manufacturing requirements and explore partnership opportunities.