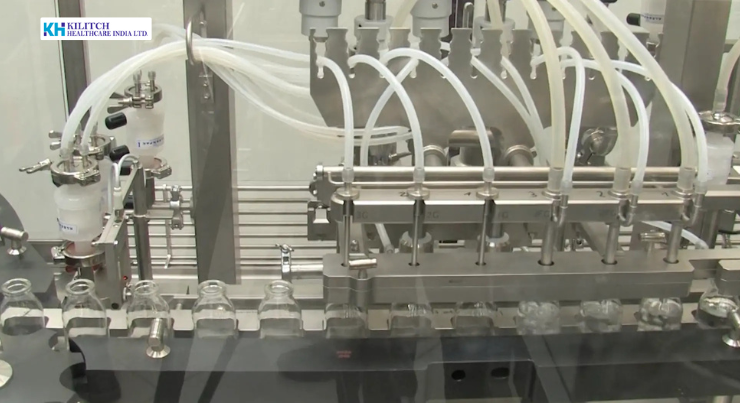
In contemporary healthcare, sterile injectables are essential for acute care, oncology, anesthesia, critical therapies, and emergency medicine. These dosage forms are delivered directly into the human body, leaving no margin for microbial contamination, particulate matter, or process variability. As a result, sterile injectable filling has become one of the most technically demanding operations within pharmaceutical manufacturing.
For pharmaceutical companies, contract manufacturing partners, and quality professionals, the challenge is not only to maintain sterility but to do so consistently, efficiently, and in full compliance with global regulatory expectations. As pharmaceutical manufacturers in India expand their presence in regulated international markets, the precision and reliability of sterile filling operations are increasingly central to both compliance and competitiveness.
This article explores the technical fundamentals of sterile injectable filling, the regulatory frameworks that govern it, the technologies that enable it, and the business value it delivers to pharmaceutical stakeholders worldwide.
The Technical Importance of Sterile Injectable Filling
Sterile filling is the final and most critical stage in the production of injectable medicines. At this point, the product is exposed to the environment and transferred into its primary container vials, ampoules, or prefilled systems making contamination control a top priority.
Contamination Control and Aseptic Handling
The primary objective of sterile filling is to prevent the introduction of microorganisms, endotoxins, and particulates.This requires:
- Controlled cleanroom environments with defined air classifications
- Strict personnel gowning and movement protocols
- Segregated material and product flow
- Continuous environmental monitoring
Even minor deviations in air pressure, airflow patterns, or operator behavior can compromise sterility. Therefore, aseptic handling is not merely a procedural requirement; it is a discipline supported by facility design, equipment engineering, and operator training.
Precision in Dosage and Product Integrity
Beyond sterility, injectable products demand high accuracy in fill volumes. Dosage precision affects drug potency, stability, and therapeutic performance. Variability at micro-level volumes can result in non-compliance, batch rejection, or downstream regulatory scrutiny.
In advanced sterile operations, filling accuracy is maintained through calibrated equipment, in-process controls, and real-time monitoring ensuring that every unit meets defined specifications.
Compliance and Standards: The Regulatory Framework Behind Sterile Operations
Sterile injectable manufacturing is governed by some of the most stringent regulatory standards in the pharmaceutical sector. Adherence to GMP good manufacturing practices is mandatory across all phases of production, from facility design to batch release.
Implementing GMP in Sterile Manufacturing
Under gmp good manufacturing practices, sterile operations must demonstrate:
- Cleanroom classification and qualification
- Equipment validation and process verification
- Documented standard operating procedures (SOPs)
- Personnel training and qualification programs
- Deviation management and corrective actions
These systems ensure that sterility is built into the process rather than tested into the final product. For pharmaceutical companies engaged in pharmaceutical contract manufacturing, robust GMP systems are essential to protect brand integrity and regulatory standing.
Quality Control Systems and Regulatory Alignment
Effective sterile filling is supported by comprehensive quality control for pharmaceutical industry practices. This includes:
- In-process controls for fill volume and container integrity
- Microbiological monitoring of environments and surfaces
- Visual inspection for particulate matter, seal defects, and container damage
- Batch documentation and traceability
Facilities that integrate quality into every operational layer are better positioned to meet inspections, audits, and market authorization requirements particularly in export-driven manufacturing models.
Equipment and Technology in Sterile Injectable Filling
Modern sterile filling operations rely on advanced equipment and automation to reduce human intervention and improve process reliability.
Automated Filling and Sealing Systems
Automated filling lines are designed to deliver precise volumes under aseptic conditions. Key technical features include:
- Closed filling systems to minimize exposure
- Servo-controlled dosing mechanisms for volumetric accuracy
- Integrated container handling to reduce manual contact
- Automated sealing to ensure container closure integrity
These systems support both high-throughput commercial production and smaller batch manufacturing for specialized therapies.
Validation, Monitoring, and Process Control
All critical equipment must undergo installation, operational, and performance qualification. Process validation confirms that sterile filling consistently produces output meeting predefined specifications.
Advanced monitoring systems track environmental parameters, machine performance, and product attributes in real time. This data-driven approach enhances both compliance and operational transparency key factors for pharmaceutical partners and regulatory bodies alike.
Business Benefits of High-Quality Sterile Filling Operations
While technical excellence and regulatory compliance are foundational, sterile injectable filling also delivers measurable business value for pharmaceutical companies and healthcare partners.
Product Quality and Market Confidence
Consistent sterile filling protects product integrity, reduces recalls, and strengthens confidence among healthcare providers and procurement teams. For companies supplying hospitals and institutional markets, this reliability is critical to long-term relationships and repeat business.
Scalability and Operational Efficiency
Automated and validated sterile filling lines enable manufacturers to scale production without compromising quality. This scalability is particularly valuable for:
- Product launches in new therapeutic areas
- Institutional tenders requiring large volumes
- Export-driven manufacturing for international markets
For pharmaceutical companies leveraging pharmaceutical contract manufacturing, scalability translates into faster time to market and improved supply chain resilience.
Cost-Effectiveness Through Process Optimization
Although sterile operations require significant infrastructure, optimized workflows and automation reduce waste, rework, and downtime. Over time, these efficiencies improve cost structures making high-quality sterile manufacturing commercially sustainable.
Kilitch Healthcare’s Approach to Sterile Injectable Manufacturing
Within India’s evolving pharmaceutical landscape, Kilitch Healthcare (KHIL) has developed manufacturing capabilities aligned with global expectations for sterile operations. With dedicated facilities, validated processes, and a quality-driven operating model, KHIL supports pharmaceutical organizations seeking reliable manufacturing partnerships.
By integrating advanced equipment, GMP-compliant quality systems, and trained technical teams, Kilitch Healthcare provides:
- Controlled sterile filling environments
- End-to-end compliance with regulatory standards
- Scalable production for domestic and international markets
- Structured quality control for pharmaceutical industry requirements
This approach enables pharmaceutical companies to outsource critical manufacturing functions while maintaining confidence in product quality, regulatory readiness, and supply reliability.
Strategic Considerations for Pharmaceutical Decision-Makers
For QA/QC professionals, regulatory leaders, and operations executives, selecting a sterile manufacturing partner is a strategic decision with long-term implications. Key evaluation criteria include:
- Depth of GMP implementation and audit readiness
- Level of automation and contamination control
- Validation and documentation practices
- Capacity for scale and market expansion
As pharmaceutical manufacturers in India continue to strengthen their global footprint, partnerships built on technical rigor and compliance excellence will define competitive advantage.
Conclusion: Strengthening Pharmaceutical Manufacturing Through Sterile Excellence
Sterile injectable filling represents the intersection of precision engineering, regulatory compliance, and patient safety. In a healthcare environment where injectable therapies are indispensable, the quality of sterile manufacturing directly influences clinical outcomes and brand credibility.
By investing in advanced technology, robust quality systems, and disciplined operational practices, pharmaceutical companies can ensure that every unit delivered to the market meets the highest standards of safety and performance. With its focus on compliance, scalability, and technical integrity, Kilitch Healthcare (KHIL) exemplifies the capabilities required for dependable sterile manufacturing in both Indian and global markets.
For organizations seeking a trusted partner in pharmaceutical manufacturing one that combines regulatory discipline with operational excellence sterile injectable filling is not just a process, but a strategic capability
To explore partnership opportunities or discuss manufacturing requirements, connect with Kilitch Healthcare’s technical team.