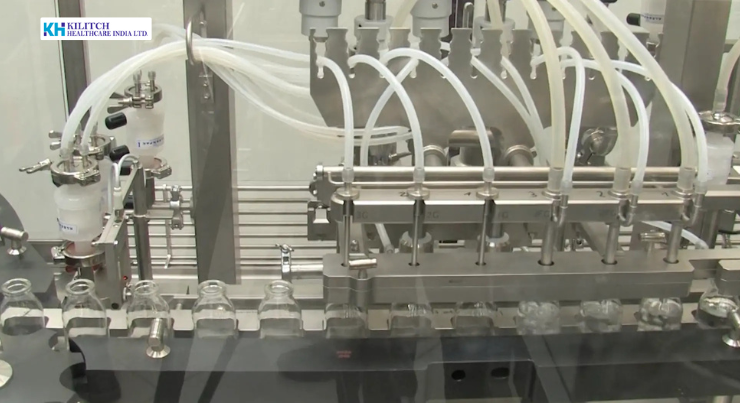
In today’s highly regulated healthcare landscape, the margin for error in pharmaceutical manufacturing is vanishingly small especially for sterile dosage forms. Sterile injectables remain a cornerstone of modern therapy in hospitals, critical care, and specialized treatment protocols. Their clinical impact is profound, but so are the technical and regulatory demands behind their production.
For pharmaceutical companies, quality leaders, and contract partners, the challenge is clear: how to deliver consistent, contamination-free, and regulation-compliant products at scale without compromising efficiency or speed to market. As the global supply of injectable medicines grows and the pharmaceutical manufacturers in India expand their footprint, precision in sterile operations has become both a competitive differentiator and a compliance imperative.
This article examines how precision, quality systems, and process efficiency converge in sterile injectable manufacturing and what decision-makers should evaluate when selecting a manufacturing partner.
Precision and Quality Challenges in Sterile Pharmaceutical Manufacturing
Sterile dosage forms are uniquely sensitive to process variability. Unlike non-sterile products, injectables are administered directly into the body, leaving no tolerance for microbial contamination, particulate matter, or dosage inconsistency. Achieving uniformity across every unit is therefore not just a technical objective; it is a patient safety mandate.
Microbiological Control and Contamination Risk
The primary challenge in producing sterile injectables is maintaining aseptic conditions throughout the manufacturing chain. Even minute lapses in environmental control, material handling, or operator procedures can compromise batch integrity. Common risk areas include:
- Inadequate air filtration or pressure differentials
- Personnel movement within critical zones
- Inconsistent sterilization of components and equipment
- Improper material flow and gowning protocols
Precision in sterile operations requires layered controls engineering, procedural, and human to ensure that contamination risks are identified and mitigated at every step.
Dosage Accuracy and Product Uniformity
In injectable manufacturing, volumetric accuracy directly influences therapeutic outcomes. Variations at the microliter level can affect drug potency, stability, and safety. This places extraordinary demands on filling equipment calibration, in-process controls, and inspection systems.
For pharmaceutical companies operating in regulated markets, failure to meet dosage specifications can result in batch rejections, regulatory observations, or product recalls making precision an operational and reputational priority.
The Technical Process: From Aseptic Preparation to Final Inspection
Achieving consistent quality in sterile manufacturing depends on a tightly controlled sequence of validated processes. While specific technologies vary by facility, best-in-class operations follow a structured approach to minimize risk and maximize repeatability.
Aseptic Preparation and Component Sterilization
The manufacturing cycle begins with the preparation of the drug solution and the sterilization of all contact components, including containers, closures, and transfer lines. Filtration systems, sterilization cycles, and material staging areas are designed to maintain product integrity before filling begins.
At this stage, adherence to GMP good manufacturing practices ensures that materials, equipment, and documentation meet defined quality standards prior to entering controlled environments.
Precision Filling and Sealing Operations
Filling is the most critical step in sterile injectable manufacturing. Automated systems are used to dispense precise volumes into ampoules, vials, or other primary containers under aseptic conditions. Key process controls include:
- Real-time monitoring of fill volumes
- Pressure and airflow management in critical zones
- Automated container handling to minimize human intervention
- Sealing technologies that ensure container integrity
These systems are designed to deliver consistent dosage across high-volume production while maintaining sterility.
Visual Inspection and Defect Detection
Post-filling, each unit undergoes inspection to identify defects such as particulate matter, container cracks, or improper seals. Advanced inspection systems often combining automated and manual verification are employed to ensure that only compliant products proceed to packaging and release.
This inspection phase is essential not only for quality assurance but also for regulatory defensibility, particularly in export-oriented operations.
Regulatory and Quality Standards: Compliance as a Core Operating Principle
In sterile manufacturing, regulatory compliance is inseparable from operational excellence. Manufacturing partners must demonstrate adherence to national and international guidelines, supported by robust quality management systems.
Implementing GMP Across the Manufacturing Lifecycle
GMP good manufacturing practices govern every aspect of sterile production from facility design and equipment qualification to documentation, training, and batch release. Core elements include:
- Cleanroom classification and environmental monitoring
- Equipment validation and maintenance protocols
- Standard operating procedures (SOPs) for aseptic handling
- Deviation management and corrective action systems
For pharmaceutical companies engaged in pharmaceutical contract manufacturing, these systems provide assurance that outsourced production meets regulatory expectations across markets.
Quality Control, Documentation, and Audit Readiness
Beyond physical controls, compliance depends on comprehensive documentation and traceability. Batch records, validation reports, stability data, and audit trails form the backbone of regulatory interactions. Facilities that integrate quality into daily operations rather than treating it as a checkpoint are better positioned to withstand inspections and sustain long-term partnerships.
For global pharmaceutical brands, this audit readiness is a non-negotiable requirement when evaluating manufacturing partners in India or abroad.
Efficiency, Scalability, and Business Impact
While precision and compliance are foundational, modern pharmaceutical operations must also deliver efficiency and scalability. The ability to meet growing demand without compromising quality is critical for both market competitiveness and supply chain reliability.
Automation and Throughput Optimization
Automation reduces human intervention in critical zones, lowering contamination risk while increasing throughput. Integrated filling, inspection, and packaging lines enable higher batch volumes with consistent output supporting rapid scale-up for product launches or institutional supply contracts.
For pharmaceutical companies navigating variable market demand, these efficiencies translate into shorter lead times, reduced waste, and improved cost control.
Supply Chain Reliability for Institutional and Export Markets
Hospital procurement teams and healthcare distributors require predictable supply and batch-to-batch consistency. Manufacturing operations that emphasize process standardization and capacity planning provide the reliability needed for institutional tenders and international distribution.
In this context, India’s growing role in global pharmaceutical manufacturing is closely tied to its ability to deliver high-quality injectables at scale supported by strong regulatory frameworks and advanced manufacturing infrastructure.
Partnering for Precision: Kilitch Healthcare’s Manufacturing Approach
Within India’s evolving pharmaceutical landscape, Kilitch Healthcare (KHIL) has established itself as a dependable partner for companies seeking technically advanced, compliance-driven manufacturing solutions. With dedicated sterile facilities and a strong commitment to quality systems, KHIL supports pharmaceutical organizations across domestic and international markets.
By aligning manufacturing processes with global regulatory expectations and investing in controlled environments, validated workflows, and trained technical teams, Kilitch Healthcare enables partners to achieve:
- Consistent quality across sterile product lines
- Scalable capacity for growing market demand
- Regulatory readiness for audits and market authorizations
This approach positions KHIL as a strategic collaborator in pharmaceutical contract manufacturing, particularly for organizations that prioritize precision, reliability, and long-term supply assurance.
Strategic Implications for Pharmaceutical Decision-Makers
For quality leaders, regulatory professionals, and operations executives, sterile manufacturing decisions have far-reaching consequences. The right manufacturing partner can accelerate time to market, strengthen compliance posture, and protect brand integrity. Conversely, gaps in process control or documentation can expose organizations to operational risk and regulatory scrutiny.
When evaluating manufacturing partnerships, decision-makers should consider:
- The maturity of quality management systems
- The level of automation and contamination control
- The facility’s track record in regulated markets
- The ability to scale without compromising standards
In an environment where injectable therapies are central to patient care, precision is not merely a technical attribute, it is a strategic advantage.
Conclusion: Advancing Precision in Pharmaceutical Manufacturing
Sterile injectable manufacturing represents one of the most demanding disciplines within the pharmaceutical industry. Success requires the seamless integration of precision engineering, rigorous quality systems, and operational efficiency supported by unwavering compliance with regulatory standards.
As global demand for injectable therapies continues to rise, pharmaceutical companies and contract partners must prioritize manufacturing models that deliver accuracy at every milliliter. With advanced infrastructure, validated processes, and a quality-first philosophy, Kilitch Healthcare (KHIL) exemplifies the capabilities required to meet these expectations in India’s expanding pharmaceutical sector.
For organizations seeking a manufacturing partner that combines technical rigor with business reliability, the path forward begins with precision, compliance, and collaboration.
To explore sterile injectable manufacturing capabilities or discuss partnership opportunities, connect with Kilitch Healthcare’s technical team.